Brazing,Induction Brazing,Brazing Aluminum,Leak Checking,Vacuum Brazing Furnace Dongguan Jeek Precision Technology Co.,LTD. , https://www.jeeklead.com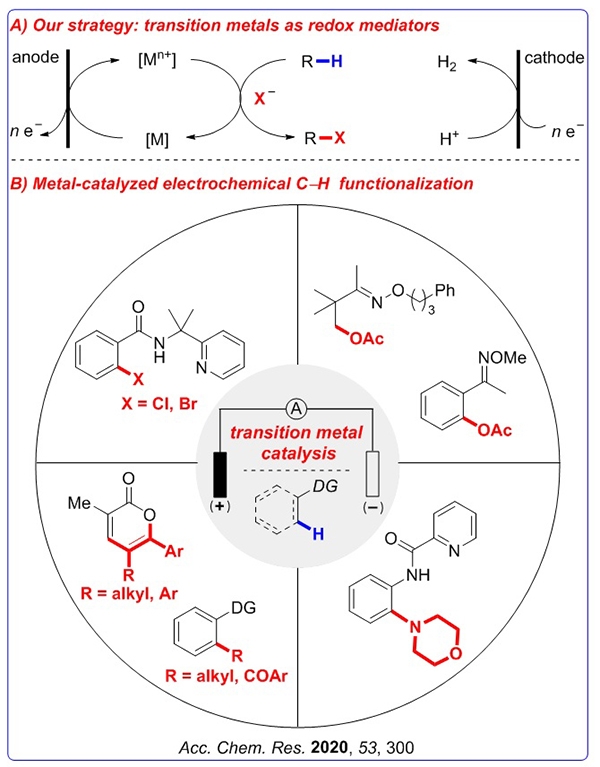
Advances in Metal Organic Electrochemical Reactions of Shanghai Institute of Organic Chemistry
[ Instrument Network Instrument R & D ] Synthetic chemistry provides a material basis for medicine, pesticides, materials, etc. required by human development. Green chemistry has also become the core concept of future synthetic chemistry, and its purpose is to minimize the impact of the synthetic process on the environment from the root and source. Redox reactions are basic chemical reactions that usually require the use of equivalent amounts of chemical oxidants or reducing agents that cause large amounts of by-products. Organic electricity synthesis uses electric energy to drive the reaction, and does not require chemical oxidants or reducing agents. It is a green synthesis technology.
Compared with traditional synthesis methods, electrosynthesis has the following three characteristics: 1) the current and potential in the electrosynthesis can be precisely controlled; 2) the electrode reaction rate can be controlled by adjusting the potential; 3) the electrosynthesis is in the same reaction system. An oxidation reaction occurs at the anode and a reduction reaction occurs at the cathode. Based on these characteristics of electrochemical reactions, electrosynthesis has been widely used not only in the industrial synthesis of inorganic compounds such as chlor-alkali industry, strong oxidants, highly active metal oxides, but also in the preparation of organic compounds. For example, as early as 1965, Monsanto (Monsanto) has built an electrosynthesis plant of 15,000 tons / year acrylonitrile electroreduction to obtain adiponitrile. In addition, about 250 kinds of organic fluorine reagents are also obtained by electrochemical oxidation and fluorination. However, traditional organic electrochemical reactions often pass through free radical intermediates, and the control of chemoselectivity and stereoselectivity is a challenge.
Mei Tiansheng's group at the State Key Laboratory of Metal Organic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences has been working in the field of metal organic electrochemistry: using transition metals as electrocatalysts to regulate electron transfer strategies and exploring chemical options in traditional electrochemical reactions It is difficult to control the selectivity, regioselectivity and stereoselectivity. They found that the strategy of using transition metals as electrocatalysts was very successful and provided a new strategy for solving the problem of traditional electrochemical selective control.
Coupling reaction The coupling reaction is a 2A-B → AA type reaction. It is a process in which an organic molecule is obtained by a certain chemical reaction between two organic chemical units. Coupling reactions have multiple pathways and are widely used in organic synthesis. The reaction of combining amino acids into a protein is also a coupling reaction.
In organic analysis, the color produced by the coupling reaction is often used to identify drugs with phenol or arylamine structures.
In the coupling reaction promoted by electroreduction, Mei Tiansheng's group used cathodic reduction to replace reducing agents in traditional chemistry, such as manganese, zinc and other metal reagents, to achieve reductive coupling reactions. Using the "pairwise electrolysis" strategy, nickel-catalyzed thioetherification of (hetero) aryl bromides or chlorides at room temperature is achieved (Angew. Chem. Int. Ed. 2019, 58, 5033-5037). Compared with the traditional C-S coupling reaction (usually above 80 ° C) assisted by the addition of alkali, this reaction provides an effective method for constructing aryl sulfides under mild conditions, without the need for an additional base and with good functional group compatibility. method. Recently, the research group adopted a migration coupling strategy to achieve the nickel-catalyzed migration coupling reaction of aryl halides and alkyl halides, providing a new way for the green synthesis of 1,1-diaryl compounds (Angew Chem. Int. Ed. 2020, DOI: 10.1002 / anie.201912753).
When performing the coupling reaction, the acidity and basicity of the medium are important. Generally, the coupling reaction between diazonium salt and phenols is carried out in a weakly alkaline medium. Under these conditions, phenol forms phenoxy anions, which increases the density of the aromatic ring electron cloud, which is conducive to the coupling reaction. The coupling reaction between the diazonium salt and the aromatic amine is carried out in a neutral or weakly acidic medium. Under these conditions, the aromatic amine forms an ammonium salt and increases its solubility. The salt-forming reaction is reversible. With the consumption of the aromatic amine in the coupling reaction, the salt of the aromatic amine will be converted back to the aromatic amine to meet the needs of the reaction. If the solution is too acidic, the amine becomes an ammonium salt, which reduces the aromatic amine concentration and weakens or suspends the coupling reaction. From the point of view of the nature of the diazonium salt, a strong alkaline medium will cause the diazonium salt to be converted into another compound that cannot undergo a coupling reaction.
Mei Tiansheng's group also developed a series of metal-catalyzed carbon-hydrogen bond selective functionalization reactions promoted by electro-oxidation to achieve the selective oxidation of C (sp3) –H bonds in alkanes (J. Am. Chem. Soc. 2017 , 139, 3293) and selective amination, acylation, alkylation, halogenation, etc. of aromatic C (sp2) –H bonds (J. Am. Chem. Soc. 2018, 140, 11487; ACS Catal. 2018, 8, 7179; Org. Lett. 2017, 19, 2905; Chem. Commun. 2017, 53, 12189). Since the bond energy of olefin carbon-hydrogen bonds is higher than that of p-bonds, it is a challenge to selectively realize the electro-oxidation of olefin carbon-hydrogen bonds.
The electro-oxidation method is a method for oxidizing substances by electrolytic means. It can be used to achieve chemical reactions that are difficult to be directly oxidized by oxygen under general conditions. By applying an overpotential (essentially reducing the activation energy of the reaction), the reaction that was originally performed at a higher temperature can be performed in a battery at room temperature.
It has many advantages: â‘ can reach a higher degree of oxidation. â‘¡ The product is relatively pure and easy to purify, and there is no pollution caused by direct use of oxidant. â‘¢ By changing the voltage and other conditions, stepwise oxidation reactions can be performed, and sometimes multi-step chemical reactions can be completed in the electrolytic cell at one time. â‘£ High selectivity. In industry, it can be used to prepare many important oxidants such as Cl2, KMnO4, KClO3, H2O2, etc. The electrochemical oxidation of aluminum and its alloys can change the properties of aluminum products and make them have good heat resistance, insulation and corrosion resistance. The insulation and thermal insulation properties make it widely used in aerospace, aviation, electrical, and electronics industries. And because its surface oxide film has many micropores and strong adsorption capacity, it can be dyed into a variety of bright and eye-catching colors, which makes it more and more widely used in light industry and architectural decoration.
Recently, the research group used metal iridium as an electrocatalyst to achieve the first selective cyclization of olefin C (sp2) –H bond promoted by electrooxidation. Compared with cyclization reactions catalyzed by cobalt or ruthenium using chemical oxidants, the reaction has better regioselectivity. It is worth pointing out that the Ir (I) complex in the cyclized intermediate has a stable coordination-saturated 18-electron structure and is not easily oxidized by a chemical oxidant to the corresponding Ir (III). Under the condition of electrooxidation, the Ir (I) complex can be easily oxidized to Ir (III). This study further illustrates the controllability and adjustability of the electrochemical oxidation potential, which can achieve some transformations that are not easily achieved using traditional chemical oxidants.
Source: Encyclopedia, Shanghai Institute of Organic Chemistry