Bronze Bushing Material advantages: Nordberg Gp Bronze Bushing,Nordberg Gp300 Bronze Bushing,Nordberg Crusher Bronze Bushing,Nordberg Gp Bronze Bushing Parts Ma An Shan Shi Bo Da Jing Shen Machinery Co.,ltd , https://www.bodamachinery.com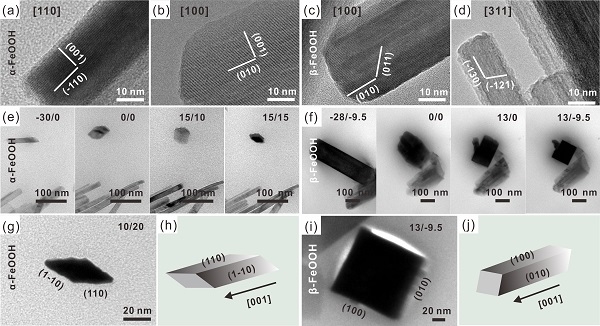
High hardness, excellent wear resistance, galling easy to produce, has good castability and machinability, good corrosion resistance in the atmosphere and fresh water.
Work wear parts can be used under a high load (20Mpa or less) and high sliding speeds (8m / s).
Corrosion resistance,Has good lubricating properties and wear resistance and corrosion resistance, suitable for use as a bimetal casting material.
High surface pressure, but also side pressure bearing, impact resistant negative Heda 50Mpa parts.
Progress in FeOOH crystal phase structure and electrochemical analysis behavior
[ Instrument Network Instrument R & D ] Recently, researcher Huang Xingjiu, a researcher in the Institute of Intelligent Machinery of the Chinese Academy of Sciences, has a postdoctoral research team of Meng Huang and associate researcher Lin Chuhong, and studied the transmission mode of FeOOH nanorods with different crystal phases using transmission electron microscope (TEM) rotation mode Cross-section structure and clarify its crystal growth direction; combined with synchrotron radiation X-ray absorption fine structure (XAFS) technology and kinetic simulation calculations, revealed its new mechanism of dominant crystal phase in electrochemical analysis of heavy metal ions.
X-ray is an electromagnetic wave with extremely short wavelength and high energy. The wavelength of X-ray is shorter than the wavelength of visible light (about 0.001 to 10 nanometers, and the wavelength of X-rays used in medicine is about 0.001 to 0.1 nanometers). Its photon energy is tens to hundreds of thousands of times greater than that of visible light. Discovered by German physicist WK Roentgen in 1895, it is also called Roentgen ray.
Crystal phase is a crystalline microstructure, which is determined by the conformation and arrangement of polymer chains in the crystal. The types are: single crystal, spherulite, dendritic crystal, twin crystal, straight-chain lamellar crystal, fibrous crystal, and string crystal. Wait. The main research tools are: optical microscope, electron microscope, etc. Among them, the content with more content is called the main crystal phase, and the content with less content is called the secondary crystal phase or the second crystal phase. Sometimes, a second crystal phase may be found on the grain boundary, and its existence generally needs to be determined by X-ray structure analysis such as X-ray diagram, energy spectrum analysis, and lattice fringe image. The crystals of ceramic materials are mainly single oxides (such as Al2O3, MgO) and composite oxides (such as spinel MgO · Al2O3, lead zirconate titanate Pb (Zr, Ti) O3). In addition, non-oxide ceramic materials also include crystals of corresponding components such as carbides, nitrides, borides, and silicides. The performance of ceramic materials is closely related to the type, number, distribution and defect status of the main crystal phase.
Metal hydroxide nanomaterials are widely used in the fields of energy and environment due to their excellent catalytic properties. Although it has been reported that the different crystal phase structures of metal hydroxide nanomaterials exhibit unique electrochemical properties, due to the complexity of the catalytic reaction system and many factors affecting its electrocatalytic properties, the crystal phases of metal hydroxide nanomaterials are numerous. The key factors behind the dependent electrochemical behavior are difficult to identify. On the one hand, there is a lack of understanding of the interaction between nanomaterials and target analytes at the atomic level; on the other hand, the kinetic processes in catalytic reactions are not clear. In addition, there is a lack of direct evidence for the internal structure and growth direction of metal hydroxide nanomaterials with different crystalline phases. Elucidating the relationship between the structure and electrochemical performance of nanomaterials has important guiding significance for designing effective sensitive interfaces.
The hydroxide is generally alkaline, but the basicity is different. It depends on the electronegativity of the corresponding element of the metal cation combined with hydroxide. The smaller the electronegativity, the easier the hydroxide is in the solution. Hydroxide is released, the stronger the alkalinity. Conversely, the greater the electronegativity, the easier it is for the hydroxide to release hydroxide in solution, and the weaker the basicity.
Based on the above problems, the researchers first synthesized rod-shaped α- and β-FeOOH, studied the cross-sectional shape by rotating the transmission electron microscope at different angles, and combined the analysis results of the lattice fringe spacing and the corresponding crystal plane of nano-diffraction. It was confirmed that the cross section of the α-FeOOH nanorods was rhombic, while the cross section of the β-FeOOH nanorods was square, and the growth directions of the α- and β-FeOOH nanorods were both [001] directions. Then, the α- and β-FeOOH nanorods were used to construct sensitive interfaces to study the electrochemical analysis behavior. The detection results showed that the detection sensitivity of α-FeOOH to Pb (II) was about 17 times higher than that of β-FeOOH. Combining synchrotron radiation XAFS technology and kinetic simulation calculations to clarify the detection signal enhancement mechanism of the dominant crystal phase α-FeOOH nanorods during electrochemical analysis. XAFS and infrared spectroscopy (FT-IR) research results show that compared with β-FeOOH, more surface hydroxyl groups are found on α-FeOOH, which helps to enrich the target analyte Pb (II), and The longer Pb-O bond adsorbed on α-FeOOH promotes the diffusion of Pb (II) to the electrode surface to undergo a redox reaction, thereby obtaining an enhanced electrochemical signal. The results of kinetic process simulation calculations clearly clarified that the adsorption rate and capacity of α- and β-FeOOH for the target analyte are the determinants of the enhanced crystalline phase's enhanced electrochemical sensitivity.
Electrochemistry is the science that studies the phenomenon of charged interfaces formed by two types of conductors and the changes that occur on them. The interaction between electrical and chemical reactions can be completed by batteries, or by high-voltage electrostatic discharge (such as the conversion of oxygen into ozone through silent discharge tubes), which are collectively referred to as electrochemistry, which is a branch of electrochemistry, called discharge chemistry . Because of the special name for discharge chemistry, electrochemistry often refers specifically to the "science of batteries."
Electrochemistry has now formed a number of branches such as synthetic electrochemistry, quantum electrochemistry, semiconductor electrochemistry, organic conductor electrochemistry, spectral electrochemistry, and bioelectrochemistry.
The research findings of this work have extended the understanding of FeOOH structure of different crystal phases on the one hand, and provided an experimental basis for its surface atomic arrangement model; on the other hand, revealed a new mechanism of sensitivity enhancement of dominant crystal phase electrochemical analysis behavior on the atomic level It provides effective theoretical support for the research of new nanomaterials for electrochemical analysis and detection and the functional application of other metal hydroxide nanomaterials.